This month I wanted to take a data-driven look at FDA’s treatment of citizen petitions, and specifically as a starting point how quickly the agency resolves those petitions. Make no mistake, I have an interest in this topic. Over the more than 35 years I have been practicing law, I have filed multiple petitions including a 1995 petition that successfully caused FDA to adopt Good Guidance Practices. But more recently, specifically on February 6, 2023, I filed a citizen petition asking FDA to rescind its final guidance on Clinical Decision Support Software.[1] On August 5, 2023, when we passed the 180 day mark, I got curious as to when I should expect a resolution. Unfortunately, the data tells me that I may well die before I hear back. I’m not kidding.
Findings
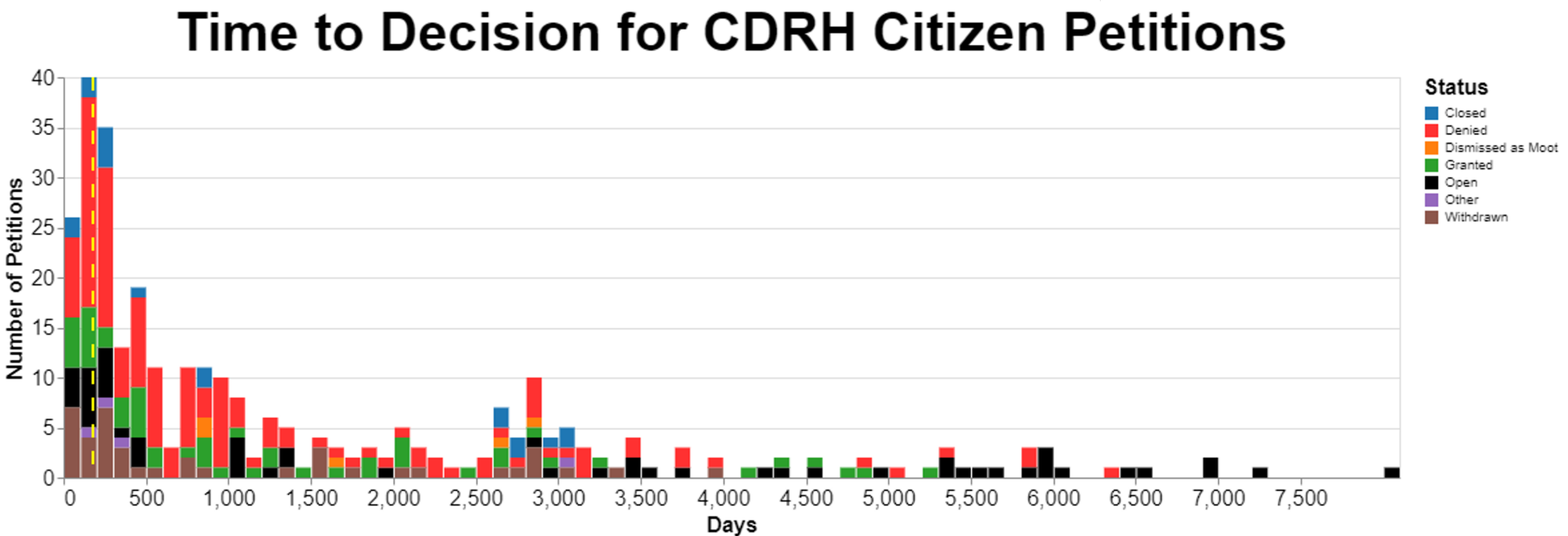
The following chart summarizes the amount of time it takes for the Center for Devices and Radiological Health (“CDRH") to reach final decisions on citizen petitions. The x-axis is very wide, so I recommend clicking on the image below to see a larger view of it.
The status is based, I’m embarrassed to say, on reading a substantial number, about 1/3, of the outstanding petitions. I’m embarrassed because as a data scientist I should’ve been able to figure out a reliable way to have software do the reading. However, I couldn’t get to a level of accuracy that I felt good enough so I did it the old-fashioned way and took an entire afternoon reading over 120 FDA decisions that my software had failed to classify with appropriate confidence.
The image is a bit garish regarding colors, but I did that because given the small font, I wanted to use distinctly different colors rather than colors that might be confused with each other. The vertical broken yellow line marks 180 days.
The categories are mine, but they’re based very closely on the language of the FDA decisions and the language of the citizen petition regulation, but I should explain them as follows:
- “Closed” is a term that FDA uses in deciding petitions where technically it isn’t denying the petition, but the agency concluded, for example, that the topic is not appropriate for a citizen petition. An example was where the FDA receive something that they considered to be a trade complaint framed as a citizen petition, but the agency wanted to treat it as a trade complaint. Closed also includes some cases where the agency deemed the request to be one for an advisory opinion, and they gave an advisory opinion in the response, and then deemed the matter closed. Thus, the agency didn’t really accept or reject the petition on policy grounds but simply explained whatever the petitioner had asked to be explained. Another subcategory here was when the petitioner died. I told you I wasn’t kidding about dying before the petitioner hears. Intuitively, the issue lingers on after the death of the petitioner, but FDA seizes on the person's death as an excuse to close the matter.
- “Denied” is hopefully self-explanatory-- the agency rejected whatever the petition requested.
- “Dismissed as moot” was used when FDA concluded that either the relevant facts or law had changed since the petition was filed and there was no longer any reason to consider the petition. This category is expressly provided for in the regulations, specifically 21 CFR 10.30(e)((2)(iii) which says the Commissioner can dismiss a petition “if at any time the Commissioner determines that changes in the law, facts or circumstances since the date on which the petition was submitted have rendered the petition moot.”
- “Granted” is also probably self-explanatory, but I should add here that I included as granted any petition that was granted even just in part. There were several petitions where the FDA granted them in part and denied them in part, but I chose to characterize even a partial win as a win.
- “Other” just include some odd ducks that are procedurally unique.
- “Withdrawn” is probably intuitive, but the circumstances may not be. This is when the petitioner decided to withdraw the petition. However, I found what might have happen behind the scenes interesting. At least one petitioner wrote that since the FDA told him that there was no way the petitioner was going to succeed, the petitioner very grudgingly withdrew the petition. It seems at least in some cases, FDA might have used this approach if they didn’t want to take the time to write what might be a challenging denial. In a very real sense, I would treat this withdrawn category the same as denied.
Methodology
What made the post possible this month is that I discovered a new governmental API of interest, specifically an API that provides access to regulations.gov.[2] It is hosted by the government’s General Services Administration. If you’re not familiar with regulations.gov, it is the repository of all communications related to notice and comment development of policy by all federal agencies. It is not, contrary to its name, limited to regulations. The nerd in me got kind of excited at the idea of how much content was sitting in this document depository.
This month, the methodology was simple. First, I made the decision to focus on CDRH rather than all of FDA as a case study as it were, because I knew, for example, that citizen petitions in the drug world have been used for different purposes including fights between branded and generic drug companies. Indeed, citizens petitions on the drug side have already been analyzed pretty extensively.[3] Thus, I was afraid that including drug related petitions might skew the data in ways that weren’t terribly interesting.
Second, I really didn’t have to guess or otherwise interpret which petitions are related to CDRH, as that center includes a public listing of their citizen petitions with links to the location of those petitions at regulations.gov.[4]
Downloading information regarding the date filed and the date of the ultimate resolution was simple. The harder part was generalizing as to how a particular petition was resolved. I wrote an algorithm that did pretty well for about two thirds of the petitions by searching for key terms. But it didn’t work so well on about 1/3, and as I confessed above, being the novice data scientist that I am, I just read the darn things.
I should note here that CDRH is violating the law in its reporting of the data. FDA’s regulations at 21 CFR section 10.30(k) require the following:
The Division of Dockets Management will maintain a chronological list of each petition filed under this section … showing:
(1) The docket number;
(2) The date the petition was filed by the Division of Dockets Management;
(3) The name of the petitioner;
(4) The subject matter involved; and
(5) The disposition of the petition.
But CDRH does not always comply. Their list, linked above, includes items one through four but not always item 5. To get item 5, you need to follow the link provided and sometimes in the header FDA indicates the outcome, but frequently they do not and you're forced to read the agency's decision to figure out the outcome. I had to read over 120 agency letters.
This is in contrast, for example, with the tobacco center that nicely provides all five categories of information here.[5]
Next month will be harder for me, because I am going to use topic modeling to assess the topics on which these petitions were filed, and show some interesting statistics on how different topics are resolved and how quickly different topics are resolved. But that’s next month.
For this month, I was content to simply show for all CDRH petitions how quickly they get resolved over time.
Interpretation
FDA takes an enormous amount of time to resolve petitions, well past expressed regulations the agency adopted. 21 CFR 10.30(e)(2) provides that “Except as provided in paragraphs (e)(4) and (5) of this section, the Commissioner shall furnish a response to each petitioner within 180 days of receipt of the petition.” Those two exceptions address abbreviated new drug applications and petitions for stays of action, neither of which are relevant to the data here. As a result, the law requires FDA to act within 180 days. As I already said, I marked 180 days on the timeline with a broken yellow vertical line.
Looking at the data, I wanted to make certain categories stand out. The two categories were “granted” -- which I put in green -- and “open” -- which I put in black. You can see that green shows up in small quantities throughout the time period all the way past 5000 days. That's interesting, because I frankly thought that if FDA was going to grant a petition, it would do so promptly. But that's not the case. However, the black category goes on the longest and it is the most troubling in a sense, because these are petitions that have not yet been resolved. As you can see, the oldest tend to be in this category.
FDA is just sitting on petitions. Not just a little bit past 180 days. Think of the order of magnitude. 900 days would be way past 180, 5 times beyond. But we are talking about almost 45 times that. We're talking about over 8000 days.
That FDA does a poor job in reviewing citizen petitions is hardly a secret or a news flash. Many have studied it before including the referenced drug review above, but also the HHS Inspector General in July of 1998.[6] Here is the entire Summary provided in the HHS OIG report from 1998:
The FDA does not have an effective process for handling citizen petitions in a timely manner, as evidenced by a backlog of approximately 250 petitions that have not been fully answered, some dating to the 1970’s and early 1980’s. The FDA regulations require tentative or responses to citizen petitions within 180 days. The backlog of pending petitions includes issues that the petitioners believe are matters of public safety, and some have requested FDA to ban or withdraw approval of certain products. When FDA does not answer petitions in a timely manner, the public may lose confidence in the regulatory process. Because the citizen petition process is not a high priority among FDA’s various responsibilities, the agency has provided limited resources to the process, and there is little central oversight of the process across FDA program areas. Recognizing its problems with the petition process, FDA developed options during the early 1990’s to improve the process; and since the start of our review, closed out slightly more petitions than it has received. We believe, however, that more can be done to reduce the backlog.
I would take issue with the characterization in that summary that a preliminary response by day 180 meets the requirement of the regulation. The regulation says nothing of the sort.
While OIG noted that FDA might have improved a bit just while the OIG was looking, it appears that FDA slipped back into its old ways. Note that some of the petitions that OIG looked at were approximately 20 years old. That still seems to be the case. The oldest open petitions at CDRH are over 8000 days old. That's about 22 years.
Analysis
This month I want to go a little bit further than just interpreting the data and leaving it there. I'd like to put it in context. This seems to be a case of where industry is expected to “Do as FDA says, but not as FDA does.” I'll explain.
Quality System Lens
Here is what FDA says industry should do. FDA’s medical device quality system regulations – specifically Sec. 820.198(a)(1) on Complaint files – says that manufacturers shall ensure that “all complaints are processed in a uniform and timely manner.” There can be serious public health issues at stake so timeliness is important. One of the reasons that the review must be timely is that companies need to determine whether a complaint triggers the need to file a Medical Device Report under part 803 of the regulations. As it turns out, FDA does not have a sense of humor about companies failing to submit MDR's on time.[7]
I'm trying to understand FDA’s actions. Citizen petitions deal with topics of great concern to citizens, and often have significant public health implications as well as issues of legal compliance. The petitions often have merit as reflected in the fact that FDA does grant many of them, albeit thousands of days later. FDA has a regulation on its books that says that it will respond to these within 180 days. But FDA routinely blows by those deadlines not just by a factor of almost 5, but by a factor of almost 45. And at the same time, FDA brings enforcement actions against companies that fail to review and report certain incidents within 30 days.
FDA's excuse typically is based on resources, but how often does FDA accept an excuse of too few resources from a company regarding its compliance? Further, let's think about the resource issue. CDRH received just over 300 petitions in 23 years. That's just over 13 petitions a year, hardly overwhelming. FDA's quality system is completely broken.
Accountability Lens
The federal government is big on accountability when it comes to companies accused of violating regulatory requirements. The United States Sentencing Commission has published seven elements that should guide the design and execution of a compliance program to ensure the company behaves properly.[8] Companies are expected to follow these elements to avoid more severe sentences when a federal crime is discovered. Let's just look at two elements of those seven.
The 2nd element requires the appointment of a senior official who is made accountable for compliance. Accountability is a keyword there. Responding to citizen petitions is what keeps FDA accountable to the citizens. The Commissioner is supposed to not just respond, but respond in a reasoned way, giving a rationale for the agency’s decision. FDA, however, ducks any accountability by leaving a petition open endlessly – for thousands of days – without providing any rationale for its implicit rejection.
The 7th element of the guidelines requires a timely response when a problem is detected. That element speaks for itself. 20 years is not timely when issues of the public health and legal compliance by a federal agency are at stake.
Conclusion
This cannot continue. The government has studied the issue before and made recommendations, only for those recommendations to be utterly ignored by FDA. This is not a case where FDA is in a difficult position, i.e. given a mandate but without funding. In the case of CDRH, we are talking about on average 13 petitions a year, hardly an avalanche and FDA data shows that the agency has no problem quickly denying some portion of those because they don't comply with the requirements of a citizen's petition. The work to be done is entirely manageable.
This is simply a case of FDA deciding that it doesn't want to do something, for reasons that it doesn't want to articulate publicly, and the agency leadership failing to hold itself accountable.
After I filed a citizen petition in February asking the agency to rescind its CDS guidance, a professor of engineering and law at the University of Florida filed another petition on July 7, 2023 asking for the same thing, but on different legal grounds.[9] The petition raises fascinating issues of First Amendment law, but one thing that stuck out to me was footnote 95 where the petition recited that 13 law firms all concluded that the guidance went beyond its statutory authority.
Here's what makes me angry. The CDS's guidance violates the Federal Food Drug and Cosmetic Act. I know it. Thirteen other law firms that have looked at it know it. A Florida law professor knows it. And FDA knows it. They can't possibly write a decision in response to either my petition or the Florida petition explaining how the guidance complies with the law.
But FDA’s strategy in this instance is simple, and it's the same strategy they use in each case where they make a mistake and are caught by a petitioner. They will just refuse to decide the matter, likely until after I'm dead (hopefully 20 years from now). That is not how government is supposed to act, and that is not the accountability that we can and should expect.
* * * *
The Unpacking Averages® blog series digs into FDA’s data on the regulation of medical products, going deeper than the published averages. The opinions expressed in this publication are those of the author(s). Subscribe to this blog for email notifications.
[1] https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-decision-support-software
[2] https://open.gsa.gov/api/regulationsgov/
[3] https://scholarship.libraries.rutgers.edu/esploro/outputs/journalArticle/Citizen-Petitions-Long-Late-Filed-and-At-Last-Denied/991031550003904646 and https://doi.org/10.7282/T3FT8Q4X
[4] https://www.fda.gov/about-fda/cdrh-foia-how-get-records-cdrh/cdrh-petitions
[5] https://www.fda.gov/tobacco-products/products-guidance-regulations/tobacco-products-related-citizen-petitions
[6] https://oig.hhs.gov/oas/reports/phs/c9750002.pdf
[7]E.g. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/irhythm-technologies-inc-643474-05252023
[8] https://www.ussc.gov/guidelines/organizational-guidelines
[9] https://www.regulations.gov/document/FDA-2023-P-2808-0001
 Click the Chart to Enlarge
Click the Chart to Enlarge